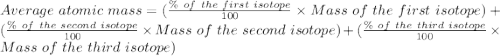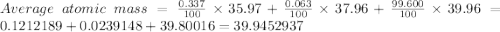Chemistry, 23.12.2019 19:31 kaydrama2003
Calculate the average atomic mass of argon to two decimal places, given the following relative atomic masses and abundances of each of the isotopes: argon-36 (35.97 u; 0.337%), argon-38 (37.96 u; 0.063%), and argon-40 (39.96 u; 99.600%)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:30
One of the following properties was originally used to arrange elements on the periodic table, but is no longer used to organize the modern version. which property fits this description?
Answers: 3

Chemistry, 21.06.2019 17:30
**40** points asapessay questions (10 points possible) clear image of next, create your own scenario. it can be one of your own real experiences or one you make up. use imagery in your writing to give your instructor a the setting and an action taking pace in your writing explain the structure and functions of the skin at work in your scenario. !
Answers: 3

Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3
You know the right answer?
Calculate the average atomic mass of argon to two decimal places, given the following relative atomi...
Questions

Mathematics, 19.05.2021 01:00




Mathematics, 19.05.2021 01:10

Mathematics, 19.05.2021 01:10

History, 19.05.2021 01:10

Mathematics, 19.05.2021 01:10


Mathematics, 19.05.2021 01:10

Mathematics, 19.05.2021 01:10



Business, 19.05.2021 01:10

Mathematics, 19.05.2021 01:10

English, 19.05.2021 01:10


Mathematics, 19.05.2021 01:10

Mathematics, 19.05.2021 01:10

Social Studies, 19.05.2021 01:10