
Chemistry, 23.12.2019 20:31 serenityarts123
Consider an ideal gas enclosed in a 1.00 l container at an internal pressure of 18.0 atm. calculate the work, w , if the gas expands against a constant external pressure of 1.00 atm to a final volume of 18.0 l.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2


Chemistry, 22.06.2019 23:30
The comparison of the number of atoms in a copper coin the size of a penny with the number of people on earth is made to illustrate which of the following? a. that atoms are indivisible b. that atoms are very small c. that atoms are very large d. that in a copper penny, there is one atom for every person on earth
Answers: 1
You know the right answer?
Consider an ideal gas enclosed in a 1.00 l container at an internal pressure of 18.0 atm. calculate...
Questions






History, 06.02.2020 02:42








History, 06.02.2020 02:42




Mathematics, 06.02.2020 02:42


Mathematics, 06.02.2020 02:42

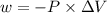
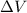 is the change in volume
is the change in volume
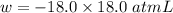
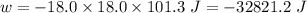 (negative sign implies that work is done by the system)
(negative sign implies that work is done by the system)

