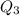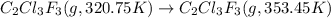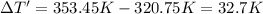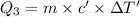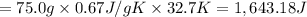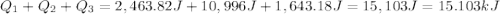Chemistry, 23.12.2019 21:31 lacyfigueroa
The fluorocarbon compound c2cl3f3 has a normal boiling point of 47.6 °c. the specific heats of c2cl3f3(l) and c2cl3f3(g) are 0.91 j/g. k and 0.67 j/g. k, respectively. the heat of vaporization for the compound is 27.49 kj/mol.
calculate the heat required to convert 75.0 g of c2cl3f3 from a liquid at 11.50 °c to a gas at 80.30 °c.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Matches the chemical name of each oxide of phosphorus to its chemical formula
Answers: 2

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2

Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

Chemistry, 23.06.2019 00:30
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1
You know the right answer?
The fluorocarbon compound c2cl3f3 has a normal boiling point of 47.6 °c. the specific heats of c2cl3...
Questions


Computers and Technology, 01.10.2019 15:00

Social Studies, 01.10.2019 15:00




Computers and Technology, 01.10.2019 15:00


Business, 01.10.2019 15:00


History, 01.10.2019 15:00

Spanish, 01.10.2019 15:00

English, 01.10.2019 15:00

Chemistry, 01.10.2019 15:00

Health, 01.10.2019 15:00

Mathematics, 01.10.2019 15:00

Health, 01.10.2019 15:00

Biology, 01.10.2019 15:00

History, 01.10.2019 15:00

Biology, 01.10.2019 15:00

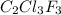 from a liquid at 11.50 °C to a gas at 80.30 °C is 15.103 kiloJoules.
from a liquid at 11.50 °C to a gas at 80.30 °C is 15.103 kiloJoules.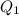
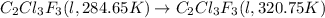
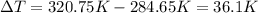
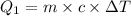
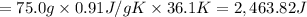
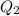
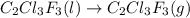
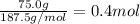
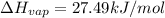
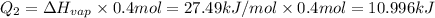
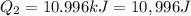 (1 kJ= 1000 J)
(1 kJ= 1000 J)