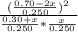Suppose a 250. ml flask is filled with 0.30 mol of n_2 and 0.70 mol of no. the following reaction becomes possible: n_2(g) +o2 → 2no(g)a. the equilibrium constant k for this reaction is 7.70 at the temperature of the flask. b. calculate the equilibrium molarity of o2. round your answer to one decimal place.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:40
During which time interval does the object travel approximately 10 meters
Answers: 3

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2


You know the right answer?
Suppose a 250. ml flask is filled with 0.30 mol of n_2 and 0.70 mol of no. the following reaction be...
Questions




Computers and Technology, 30.11.2019 04:31
















Computers and Technology, 30.11.2019 04:31