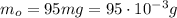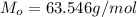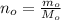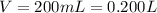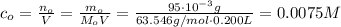Chemistry, 23.12.2019 22:31 eweqwoewoji
One way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(ii) carbonate, in concentrated sulfuric acid. the sulfuric acid reacts with the copper(ii) carbonate to produce a blue solution of copper(ii) sulfate. scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: fe(s)+cuso4(aq)→cu(s)+feso4(aq)supp ose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. he adds powdered iron to a 200.ml copper(ii) sulfate sample from the plant until no more copper will precipitate. he then washes, dries, and weighs the precipitate, and finds that it has a mass of 95.mg. calculate the original concentration of copper(ii) sulfate in the sample.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2

Chemistry, 23.06.2019 00:10
Find the missing probability in the table below a.0.10 b.40 c.0.80 d. 0.20
Answers: 2
You know the right answer?
One way in which the useful metal copper is produced is by dissolving the mineral azurite, which con...
Questions




History, 23.11.2020 22:30



Mathematics, 23.11.2020 22:30




Computers and Technology, 23.11.2020 22:30

Mathematics, 23.11.2020 22:30

Mathematics, 23.11.2020 22:30


History, 23.11.2020 22:30


Biology, 23.11.2020 22:30


Biology, 23.11.2020 22:30

History, 23.11.2020 22:30