

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1
You know the right answer?
Sodium-24 is used to treat leukemia and has a half-life of 15 hours. a patient was injected with a s...
Questions

Biology, 16.07.2019 08:00


Biology, 16.07.2019 08:00



Biology, 16.07.2019 08:00

Biology, 16.07.2019 08:00

History, 16.07.2019 08:00

Biology, 16.07.2019 08:00



Biology, 16.07.2019 08:00


Biology, 16.07.2019 08:00

Mathematics, 16.07.2019 08:00

Biology, 16.07.2019 08:00

Social Studies, 16.07.2019 08:00

Biology, 16.07.2019 08:00


Biology, 16.07.2019 08:00

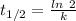
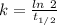
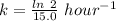
![[A_t]=[A_0]e^{-kt}](/tpl/images/0431/0796/1ef89.png)
![[A_t]](/tpl/images/0431/0796/5262c.png) is the concentration at time t
is the concentration at time t
![[A_0]](/tpl/images/0431/0796/9a686.png) is the initial concentration
is the initial concentration
![\frac {[A_t]}{[A_0]}=e^{-0.04621\times 48}](/tpl/images/0431/0796/96ef3.png)
![\frac {[A_t]}{[A_0]}=0.10881](/tpl/images/0431/0796/b1cfc.png)


