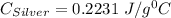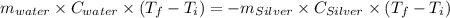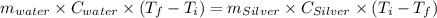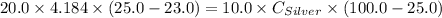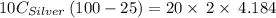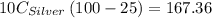Chemistry, 24.12.2019 02:31 markipler01
A10.0 gram sample of silver is heated to 100.0 degree c and then added to 20.0 g of water at 23.0 c, in an insulated container. at thermal equilibrium, the temperature of the system was measured and found to be 25.0 c. what is the specific heat, cs, of silver?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Each pair of clay balls represents to planetesimals if each plane test molluscum pound of the same material and is separated by the same distance which pair experiences the greatest gravitational attraction
Answers: 2

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2
You know the right answer?
A10.0 gram sample of silver is heated to 100.0 degree c and then added to 20.0 g of water at 23.0 c,...
Questions

History, 06.07.2020 14:01


Mathematics, 06.07.2020 14:01

English, 06.07.2020 14:01

Social Studies, 06.07.2020 14:01



Mathematics, 06.07.2020 14:01

Mathematics, 06.07.2020 14:01


Mathematics, 06.07.2020 14:01



Geography, 06.07.2020 14:01

Mathematics, 06.07.2020 14:01

Mathematics, 06.07.2020 14:01


Mathematics, 06.07.2020 14:01


Mathematics, 06.07.2020 14:01