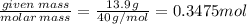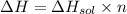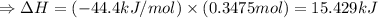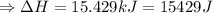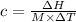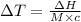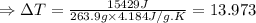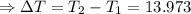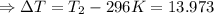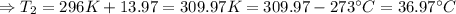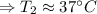Chemistry, 24.12.2019 02:31 coolman5999alt
The enthalpy of solution of sodium hydroxide is –44.4 kj/mol. when a 13.9-g sample of naoh dissolves in 250.0 g of water 23.0 °c in a coffee-cup calorimeter, what is the final temperature of the solution assuming no heat is lost to the surroundings. the solution has the same specific heat of 4.184 j/g-k.

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 23:00
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1

Chemistry, 23.06.2019 01:30
In what way do investigations build scientific knowledge? the results of investigations lead to questions that cannot be tested. they reflect the opinions and social values of scientists, ensuring valid information. the results of investigations lead to new questions, which lead to new investigations. they are not influenced by the research of earlier scientists, so they are able to address gaps in understanding.i
Answers: 1
You know the right answer?
The enthalpy of solution of sodium hydroxide is –44.4 kj/mol. when a 13.9-g sample of naoh dissolves...
Questions



English, 21.05.2021 01:00


Mathematics, 21.05.2021 01:00


Mathematics, 21.05.2021 01:00


Chemistry, 21.05.2021 01:00

Mathematics, 21.05.2021 01:00

Mathematics, 21.05.2021 01:00






History, 21.05.2021 01:00

Mathematics, 21.05.2021 01:00

Biology, 21.05.2021 01:00

Mathematics, 21.05.2021 01:00

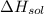 = – 44.4 kJ/mol,
= – 44.4 kJ/mol,