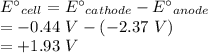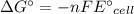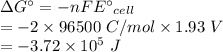Calculate the standard free-energy change at 25 ∘c for the following reaction:
mg(s)+fe2+(aq...

Chemistry, 24.12.2019 04:31 okasiafolk27
Calculate the standard free-energy change at 25 ∘c for the following reaction:
mg(s)+fe2+(aq)→mg2+(aq)+fe(s)
express your answer to three significant figures and include the appropriate units.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 08:30
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1
You know the right answer?
Questions


History, 13.05.2021 23:40

French, 13.05.2021 23:40


Computers and Technology, 13.05.2021 23:40


Mathematics, 13.05.2021 23:40



Mathematics, 13.05.2021 23:40


History, 13.05.2021 23:40


Mathematics, 13.05.2021 23:40

Computers and Technology, 13.05.2021 23:40

Social Studies, 13.05.2021 23:40



Mathematics, 13.05.2021 23:40

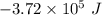
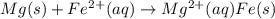
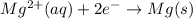 E° = -2.37 V
E° = -2.37 V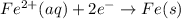 E° = -0.44 V
E° = -0.44 V