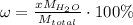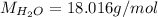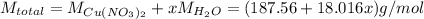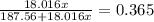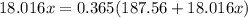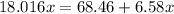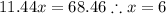Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1
You know the right answer?
The formula for hydrated copper(ii) nitrate is cu(no3)2.xh2o. it contains 36.5% water crystallizatio...
Questions

Mathematics, 10.07.2019 01:30

Mathematics, 10.07.2019 01:30


Mathematics, 10.07.2019 01:30

Mathematics, 10.07.2019 01:30

Mathematics, 10.07.2019 01:30

English, 10.07.2019 01:30

Health, 10.07.2019 01:30

Mathematics, 10.07.2019 01:30

English, 10.07.2019 01:30



Mathematics, 10.07.2019 01:30

Advanced Placement (AP), 10.07.2019 01:30




Mathematics, 10.07.2019 01:30


Mathematics, 10.07.2019 01:30