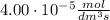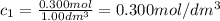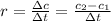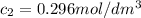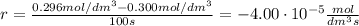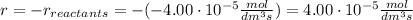Chemistry, 24.12.2019 18:31 hesterkl1225
At the start of a reaction, a 1.00 dm solution contains 0.300 mol of ethanol.
after 100 seconds the concentration of the ethanol has decreased to 0.296 mol/dmº
what is the rate of reaction over the first 100 seconds?
a 2.96 x 10-3 mol/dm/s
b 3.00 x 10 mol/dm/s
c 4.00 x 10 mol/dm®/s
d 8.00 x 10 mol/dm/s

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1
You know the right answer?
At the start of a reaction, a 1.00 dm solution contains 0.300 mol of ethanol.
after 100 seconds...
after 100 seconds...
Questions


Health, 27.08.2019 21:30

English, 27.08.2019 21:30

Mathematics, 27.08.2019 21:30


History, 27.08.2019 21:30



Mathematics, 27.08.2019 21:30

History, 27.08.2019 21:30



Mathematics, 27.08.2019 21:30

Mathematics, 27.08.2019 21:30

History, 27.08.2019 21:30


Biology, 27.08.2019 21:30