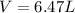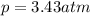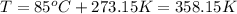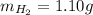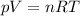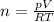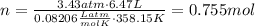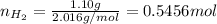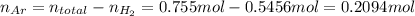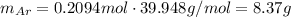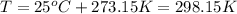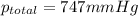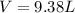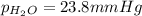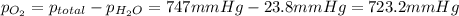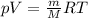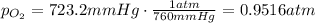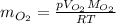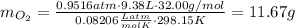Chemistry, 24.12.2019 18:31 yousifgorgees101
Amixture of hydrogen and argon gases is maintained in a 6.47 l flask at a pressure of 3.43 atm and a temperature of 85 °c. if the gas mixture contains 1.10 grams of hydrogen, the number of grams of argon in the mixture is g.
b) oxygen gas can be prepared by heating potassium chlorate according to the following equation:
2kclo3(s) > 2kcl(s) + 3o2(g)
the product gas, o2, is collected over water at a temperature of 25 °c and a pressure of 747 mm hg. if the wet o2 gas formed occupies a volume of 9.38l, the number of grams of o2 formed is g. the vapor pressure of water is 23.8 mm hg at 25 °c.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 21:50
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1

Chemistry, 23.06.2019 06:50
What is the volume of 3.2 moles of chlorine gas (cl2) at 295 k and 1.1 atm?
Answers: 1

Chemistry, 23.06.2019 09:00
What factor besides temperature affects the boiling point of water? a. mass b. number of moles c. volume d. pressure
Answers: 3

Chemistry, 23.06.2019 09:50
T(s) in2os] (m) 0 185 2.39 546 1.90 725 1.70 the decomposition of n205 can be described by the equation 2.68 given these data for the reaction at 45°c in carbon tetrachloride solution, calculate the average rate of reaction for each successive time interval. ntr s to 185 s 185 s to 546 s 546 s to 725 s number number number reaction rate: m/s m/s m/s
Answers: 1
You know the right answer?
Amixture of hydrogen and argon gases is maintained in a 6.47 l flask at a pressure of 3.43 atm and a...
Questions



Biology, 01.02.2021 22:30

Mathematics, 01.02.2021 22:30


Mathematics, 01.02.2021 22:30


Mathematics, 01.02.2021 22:30


Biology, 01.02.2021 22:30

Mathematics, 01.02.2021 22:30


English, 01.02.2021 22:30


Mathematics, 01.02.2021 22:30


Mathematics, 01.02.2021 22:30



Mathematics, 01.02.2021 22:30