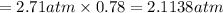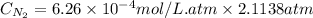As a scuba diver descends under water, the pressure increases. at a total air pressure of 2.71 atm and a temperature of 25.0 c, what is the solubility of n2 in a diver's blood? [use the value of the henry's law constant k calculated , 6.26 x 10^{-4} (mol/(l*atm).]assume that the composition of the air in the tank is the same as on land and that all of the dissolved nitrogen remains in the blood. express your answer with the appropriate units.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1


Chemistry, 22.06.2019 21:30
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3
You know the right answer?
As a scuba diver descends under water, the pressure increases. at a total air pressure of 2.71 atm a...
Questions


Mathematics, 24.07.2019 20:00


Arts, 24.07.2019 20:00


Mathematics, 24.07.2019 20:00

Advanced Placement (AP), 24.07.2019 20:00

Mathematics, 24.07.2019 20:00





Mathematics, 24.07.2019 20:00


Mathematics, 24.07.2019 20:00




Business, 24.07.2019 20:00

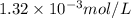 is the solubility of nitrogen gas in a diver's blood.
is the solubility of nitrogen gas in a diver's blood.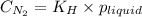
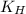 = Henry's constant =
= Henry's constant = 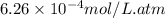
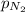 = partial pressure of nitrogen
= partial pressure of nitrogen 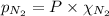 (Raoult's law)
(Raoult's law)