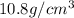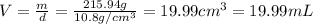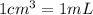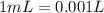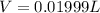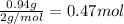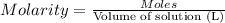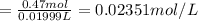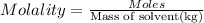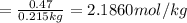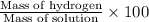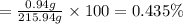Solutions of hydrogen in palladium may be formed by exposing pd metal to h gas. the concentration of hydrogen in the palladium depends on the pressure of h gas applied, but in a more complex fashion than can be described by henry’s law. under certain conditions, 0.94 g of hydrogen gas is dissolved in 215 g of palladium metal.
(a) determine the molarity of this solution (solution density = 10.8 g/cm3).
(b) determine the molality of this solution (solution density = 10.8 g/cm3).
(c) determine the percent by mass of hydrogen atoms in this solution (solution density = 10.8 g/cm3).

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:40
Which of the following is a testable hypothesis? a. if i brush my teeth, i will get fewer cavities than if i don't brush my teeth. b. green toothpaste tastes better than blue toothpaste or red toothpaste. c. smart, careful, healthy people always brush their teeth. d. it's wrong to not brush your teeth before you have an important conversation with someone.
Answers: 1

Chemistry, 21.06.2019 18:00
During which movies do spring tides new moon first quarter waxing gibbous waxing
Answers: 1

Chemistry, 21.06.2019 22:30
Often on a topographic map, every fifth contour line is darkened. what is this line called? a. key b.slope c.benchmark d. index contour
Answers: 1

You know the right answer?
Solutions of hydrogen in palladium may be formed by exposing pd metal to h gas. the concentration of...
Questions

Geography, 04.05.2021 04:30

Mathematics, 04.05.2021 04:30



Mathematics, 04.05.2021 04:30

Computers and Technology, 04.05.2021 04:30



Mathematics, 04.05.2021 04:30



Mathematics, 04.05.2021 04:30



Mathematics, 04.05.2021 04:30


Mathematics, 04.05.2021 04:30