Chemistry, 24.12.2019 20:31 smartgirl61987
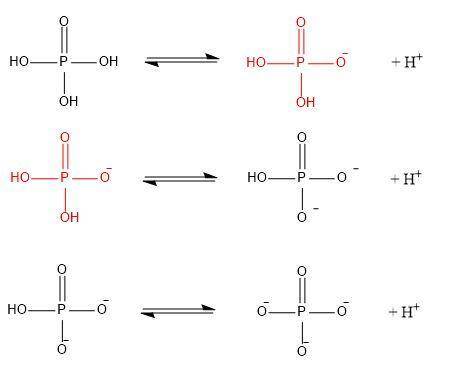
Phosphate is derived from the titration of phosphoric acid, h3po4. the three pka’s of h3po4 are 2.15, 7.20, and 12.35 respectively.
a/ write out the series of ionization (equilibrium) reactions corresponding to each ionization, making sure to write out the (flat) structure each molecule/ion as you do so. mark correct chemical bonds.
b/ in your diagram above, circle the dominant form of phosphate as it would appear at ph 5.7.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Llama have 74 chromosomes how many chromosomes will they be found in their gametes explain how you know
Answers: 2

Chemistry, 22.06.2019 00:00
Explain which group an element with the electron configuration 1s2 2s2 2p6 3s2 3p6 3d1 4s2 belongs to.
Answers: 3


Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2
You know the right answer?
Phosphate is derived from the titration of phosphoric acid, h3po4. the three pka’s of h3po4 are 2.15...
Questions

Mathematics, 07.10.2019 04:30



Business, 07.10.2019 04:30


English, 07.10.2019 04:30

Mathematics, 07.10.2019 04:30

Mathematics, 07.10.2019 04:30

Biology, 07.10.2019 04:30



Physics, 07.10.2019 04:30





History, 07.10.2019 04:30


Health, 07.10.2019 04:30