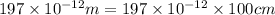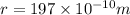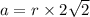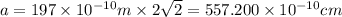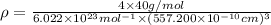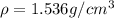Chemistry, 25.12.2019 00:31 joycetleiji1
Calcium has a cubic closest packed structure (fcc) as a solid. assuming that calcium has an atomic radius of 197 pm, calculate the density of solid calcium. (1 pm = 10-12 m, 100 cm = 1 m)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
The table lists pressure and volume values for a particular gas. which is the best estimate for the value of v at p = 7.0 × 103 pascals?
Answers: 3

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 21:30
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3

Chemistry, 22.06.2019 23:10
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium.b)heavier than helium.c)the same weight as helium.d)dependent on the element that reacted with carbon.
Answers: 3
You know the right answer?
Calcium has a cubic closest packed structure (fcc) as a solid. assuming that calcium has an atomic r...
Questions




Chemistry, 10.11.2021 22:50

English, 10.11.2021 22:50


Biology, 10.11.2021 22:50

Mathematics, 10.11.2021 22:50

English, 10.11.2021 22:50

Mathematics, 10.11.2021 22:50


English, 10.11.2021 22:50

Mathematics, 10.11.2021 22:50

Biology, 10.11.2021 22:50

Mathematics, 10.11.2021 22:50


Geography, 10.11.2021 22:50



Mathematics, 10.11.2021 22:50

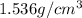 is the density of solid calcium.
is the density of solid calcium.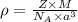
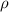 = density
= density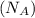 = Avogadro's number
= Avogadro's number