Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3


Chemistry, 22.06.2019 21:30
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3

Chemistry, 23.06.2019 02:20
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2
You know the right answer?
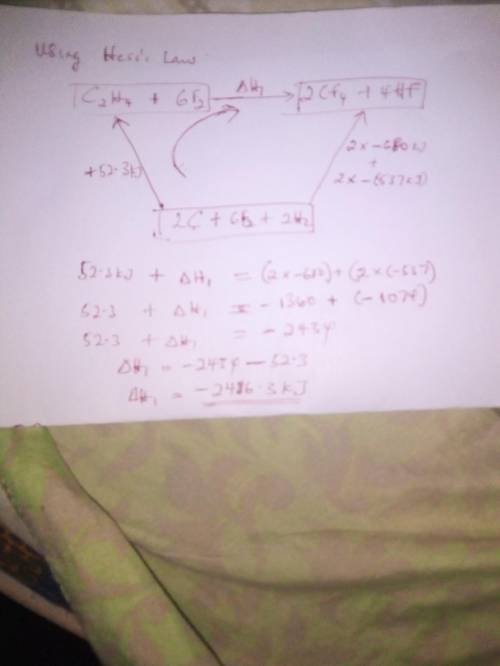
From the enthalpies of reactionh2 (g) + f2 (g) → 2 hf (g) δh = -537 kjc (s) + 2 f2 (g) → cf4 (g) δh...
Questions

Mathematics, 05.08.2019 01:30




Biology, 05.08.2019 01:30

Biology, 05.08.2019 01:30


Biology, 05.08.2019 01:30




Chemistry, 05.08.2019 01:30


Computers and Technology, 05.08.2019 01:30





Mathematics, 05.08.2019 01:30