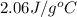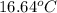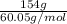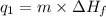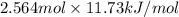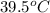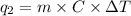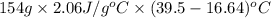Chemistry, 25.12.2019 03:31 rubianny03
Calculate the amount of heat needed to melt 154. g of solid acetic acid (hch3co2) and bring it to a temperature of 39.5 . be sure your answer has a unit symbol and the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Aspirin has a density of 1.40 g/cm3 what is the volume in cubic centimeters of a tablet weighing 320 mg ?
Answers: 1

Chemistry, 21.06.2019 19:30
Si una estrella no tiene paralaje medible, ¿qué puedes inferir?
Answers: 1

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2
You know the right answer?
Calculate the amount of heat needed to melt 154. g of solid acetic acid (hch3co2) and bring it to a...
Questions


Biology, 05.05.2020 15:49






History, 05.05.2020 15:49



Mathematics, 05.05.2020 15:49


Mathematics, 05.05.2020 15:49


Mathematics, 05.05.2020 15:49





Mathematics, 05.05.2020 15:49