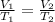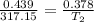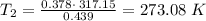Chemistry, 25.12.2019 05:31 2021CanadyRaniya
`i am holding a balloon containing 439 ml of gas over my fireplace. the temperature and pressure of the gas inside the balloon is 317.15 k and 0.959 atm, respectively. suppose i don't want the pressure to change, but i want to the volume to go down to 0.378 l. what is the temperature that i need to reach when i cool down the balloon? to what temperature (in celsius) must the balloon be cooled to reduce its volume to 0.378 l if the pressure doesn't change (remained constant)?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1

Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3

Chemistry, 23.06.2019 09:10
Complete the following radioactive decay problem. tan+on-? c+th
Answers: 1
You know the right answer?
`i am holding a balloon containing 439 ml of gas over my fireplace. the temperature and pressure of...
Questions

Mathematics, 27.05.2020 21:58

Mathematics, 27.05.2020 21:58






Chemistry, 27.05.2020 21:58

Computers and Technology, 27.05.2020 21:58

Mathematics, 27.05.2020 21:58





English, 27.05.2020 21:58

History, 27.05.2020 21:58