
Chemistry, 25.12.2019 23:31 spacehunter22
An unknown weak acid, ha, it titrated with 1.2 m naoh. the ph at the halfway point of this titration was found to be 4.102. if the initial ph of the weak acid solution (before titration) has a ph of 2.308, what was the concentration of the weak acid solution?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:50
The electron configuration for chromium is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 5 4 s 1 instead of 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 4 4 s 1 . the configuration is an exception to the
Answers: 3

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1
You know the right answer?
An unknown weak acid, ha, it titrated with 1.2 m naoh. the ph at the halfway point of this titration...
Questions



Health, 17.07.2020 17:01


Mathematics, 17.07.2020 17:01

Physics, 17.07.2020 17:01

Chemistry, 17.07.2020 17:01


History, 17.07.2020 17:01






Mathematics, 17.07.2020 17:01

Mathematics, 17.07.2020 17:01


Mathematics, 17.07.2020 17:01


Physics, 17.07.2020 17:01

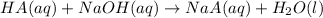
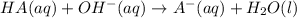
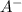 produced. That said, we have a weak acid and its conjugate base forming a buffer. We may apply the Henderson-Hasselbach equation for buffers here:
produced. That said, we have a weak acid and its conjugate base forming a buffer. We may apply the Henderson-Hasselbach equation for buffers here:![pH = pK_a + log(\frac{[A^-]}{[HA})](/tpl/images/0433/2346/a2dd4.png)
![[HA] = [A^-]](/tpl/images/0433/2346/96807.png) when half of equivalence volume of NaOH is added.
when half of equivalence volume of NaOH is added.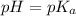 at midpoint. We may then find the acid ionization constant for this acid:
at midpoint. We may then find the acid ionization constant for this acid: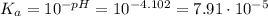
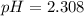
![pH = -log[H_3O^+]](/tpl/images/0433/2346/89cf7.png) , then
, then ![[H_3O^+] = 10^{-pH}](/tpl/images/0433/2346/341c1.png)
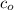 , we may write the equilibrium constant expression as:
, we may write the equilibrium constant expression as:![K_a = \frac{[H_3O^+]^2}{c_o - [H_3O^+]}](/tpl/images/0433/2346/86a10.png)
![c_o = \frac{[H_3O^+]^2}{K_a} + [H_3O^+] = \frac{10^{-2pH}}{K_a} + 10^{-pH} = \frac{10^{-2\cdot 2.308}}{7.91\cdot 10^{-5}} + 10^{-2.308} = 0.311 M](/tpl/images/0433/2346/5826b.png)


