
Chemistry, 26.12.2019 03:31 lauren21bunch
Assume that magnesium would act atom-for-atom the same as copper in this experiment. how many grams of magnesium would have been used in the reaction if one gram of silver were produced? the atomic mass of magnesium is 24.31 g/mol.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:10
Which of these is the result of scientific research and not engineering? a. a new shoe design that features air cushioning for more comfort and protection b. the creation of glass with uv protection. c. a conclusion about diet commonalities among diabetics. d. the development of a smaller, more compact missile.
Answers: 1

Chemistry, 22.06.2019 02:00
What is the maximum number of electrons that an atomic orbital can contain?
Answers: 1

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3
You know the right answer?
Assume that magnesium would act atom-for-atom the same as copper in this experiment. how many grams...
Questions




Computers and Technology, 07.04.2020 16:58







Biology, 07.04.2020 16:58


Biology, 07.04.2020 16:58







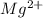 (aq) + 2Ag(s)
(aq) + 2Ag(s)


