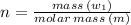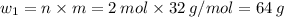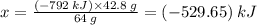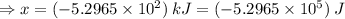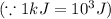Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1

You know the right answer?
Sulfur undergoes combustion to yield sulfur trioxide by the following reaction equation:
Questions

Law, 03.08.2021 22:00



Computers and Technology, 03.08.2021 22:00



English, 03.08.2021 22:00





Social Studies, 03.08.2021 22:00




Mathematics, 03.08.2021 22:00


Mathematics, 03.08.2021 22:00

Mathematics, 03.08.2021 22:00

English, 03.08.2021 22:00