
Chemistry, 26.12.2019 20:31 portedon6626
Suppose a system returns to its original overall, internal energy ((∆u) after the following changes. in step 1, 25 j of work is done on the system as it releases 37 j of energy as heat. if, in step 2, the system performs 12 j of work, how much heat is transferred?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:40
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2

Chemistry, 22.06.2019 01:30
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1


Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1
You know the right answer?
Suppose a system returns to its original overall, internal energy ((∆u) after the following changes....
Questions


Spanish, 21.11.2019 11:31

Geography, 21.11.2019 11:31


History, 21.11.2019 11:31


Mathematics, 21.11.2019 11:31

English, 21.11.2019 11:31

Mathematics, 21.11.2019 11:31


English, 21.11.2019 11:31



History, 21.11.2019 11:31

Mathematics, 21.11.2019 11:31


Mathematics, 21.11.2019 11:31



Mathematics, 21.11.2019 11:31

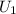 = -37 + 1.25 = -35.75 J
= -37 + 1.25 = -35.75 J

