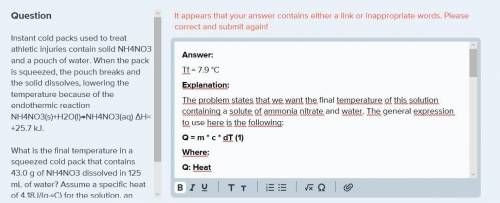
Instant cold packs used to treat athletic injuries contain solid nh4no3 and a pouch of water. when the pack is squeezed, the pouch breaks and the solid dissolves, lowering the temperature because of the endothermic reaction nh4no3(s)+h2o(l)→nh4no3(aq) δh= +25.7 kj.
what is the final temperature in a squeezed cold pack that contains 43.0 g of nh4no3 dissolved in 125 ml of water? assume a specific heat of 4.18j/(g⋅∘c) for the solution, an initial temperature of 27.5 ∘c, and no heat transfer between the cold pack and the environment.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 23.06.2019 04:00
What two categories of toxins were present in the air at dish,texas as a result of the gas pipelines that pass through the area
Answers: 1

Chemistry, 23.06.2019 15:30
Select the correct answer.the gas in a sealed container has an absolute pressure of 125.4 kilopascals. if the air around the container is at a pressure of 99.8 kilopascals, what is thegauge pressure inside the container?
Answers: 3
You know the right answer?
Instant cold packs used to treat athletic injuries contain solid nh4no3 and a pouch of water. when t...
Questions





Mathematics, 25.01.2020 13:31

History, 25.01.2020 13:31

Biology, 25.01.2020 13:31

History, 25.01.2020 13:31

Mathematics, 25.01.2020 13:31

World Languages, 25.01.2020 13:31



History, 25.01.2020 13:31





English, 25.01.2020 13:31