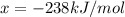Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 22.06.2019 22:30
What is the work done by the electric force to move a 1 c charge from a to b?
Answers: 2

Chemistry, 23.06.2019 07:00
0.88 moles of n2o5 (g) was placed in a sealed 1.00 l vessel. calculate the equilibrium concentration of n2o5. no2, and o2 and the equilibrium constant after equilibrium has been reached by 65.0% of the n2o5 decomposing.
Answers: 1

Chemistry, 23.06.2019 08:00
If the solubility of a gas in water is 1.22 g/l at 2.75 atm, what is its solubility (in g/l) at 1.0 atm?
Answers: 1
You know the right answer?
Consider the following reaction: a2 + b2 → 2ab δh = –321 kj bond energy (a2) = 1/2ab bond energy (b...
Questions








Mathematics, 01.01.2020 09:31


Mathematics, 01.01.2020 09:31

History, 01.01.2020 09:31

Chemistry, 01.01.2020 09:31

Mathematics, 01.01.2020 09:31


Mathematics, 01.01.2020 09:31

Mathematics, 01.01.2020 09:31

Mathematics, 01.01.2020 09:31

English, 01.01.2020 09:31


 is -238 kJ/mol
is -238 kJ/mol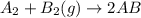

![\Delta H=\sum [n\times B.E(reactant)]-\sum [n\times B.E(product)]](/tpl/images/0434/0215/42942.png)
![\Delta H=[(n_{A_2}\times B.E_{A_2})+(n_{B_2}\times B.E_{B_2}) ]-[(n_{AB}\times B.E_{AB})]](/tpl/images/0434/0215/c021c.png)
![\Delta H=[(1\times x)+(1\times B.E_{B_2}) ]-[(2\times 2x)]](/tpl/images/0434/0215/7aa1f.png)
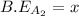
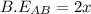
![-321=[(1\times x)+(1\times 393)]-[(2\times 2x)]](/tpl/images/0434/0215/06819.png)