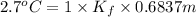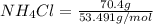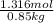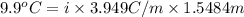When of benzamide are dissolved in of a certain mystery liquid , the freezing point of the solution is less than the freezing point of pure . calculate the mass of ammonium chloride that must be dissolved in the same mass of to produce the same depression in freezing point. the van't hoff factor for ammonium chloride in .

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 20:50
Which real-world scenarios below represent physical and chemical changes? -running a car -exploding fireworks -mixing water and powdered drink mix -combining oil and vinegar to make salad dressing -taking aspirin for a headache -diluting bleach with water-digesting dinner-spreading peanut butter on bread
Answers: 2

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3

Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3
You know the right answer?
When of benzamide are dissolved in of a certain mystery liquid , the freezing point of the solution...
Questions

Business, 19.11.2021 18:50

Social Studies, 19.11.2021 18:50

Chemistry, 19.11.2021 18:50


English, 19.11.2021 18:50

Spanish, 19.11.2021 18:50





Mathematics, 19.11.2021 19:00


World Languages, 19.11.2021 19:00


Mathematics, 19.11.2021 19:00





History, 19.11.2021 19:00

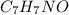 ) are dissolved in 850 g of a certain mystery liquid X, the freezing point of the solution is
) are dissolved in 850 g of a certain mystery liquid X, the freezing point of the solution is 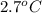 lower than the freezing point of pure X. On the other hand, when 70.4 g of ammonium chloride (
lower than the freezing point of pure X. On the other hand, when 70.4 g of ammonium chloride (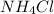 ) are dissolved in the same mass of X, the freezing point of the solution is
) are dissolved in the same mass of X, the freezing point of the solution is 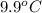 lower than the freezing point of pure X.
lower than the freezing point of pure X.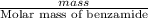
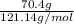
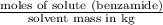
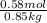
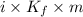 ,
, 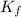 = freezing point constant of solvent
= freezing point constant of solvent