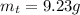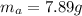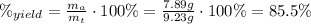Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3

Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1


Chemistry, 23.06.2019 00:50
What is the enthalpy of combustion (per mole) of c4h10 (g)? –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol
Answers: 1
You know the right answer?
Areaction with a theoretical yield of 9.23 g produced 7.89 g of product. what is the percent yield f...
Questions


English, 11.10.2020 01:01

English, 11.10.2020 01:01


Law, 11.10.2020 01:01


History, 11.10.2020 01:01


Chemistry, 11.10.2020 01:01

English, 11.10.2020 01:01

Mathematics, 11.10.2020 01:01

History, 11.10.2020 01:01



Mathematics, 11.10.2020 01:01

Mathematics, 11.10.2020 01:01

Social Studies, 11.10.2020 01:01

Mathematics, 11.10.2020 01:01

Chemistry, 11.10.2020 01:01