
Chemistry, 26.12.2019 23:31 coryowens44
1. for a hydrogen‑like atom, classify the electron transitions according to whether they result in the absorption or emission of light? n=1 to n=3, n=2 to n=1, n=3 to n=2, n=3 to n=52. ignoring sign, which transition is associated with the greatest energy change? o n=1 to n=3 o n=2 to n=1 o n=3 to n=2 o n=3 to n=5

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 03:00
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3


Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3
You know the right answer?
1. for a hydrogen‑like atom, classify the electron transitions according to whether they result in t...
Questions


Mathematics, 04.11.2020 18:10




Computers and Technology, 04.11.2020 18:10




Mathematics, 04.11.2020 18:10








Law, 04.11.2020 18:10


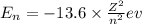
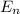 = energy of
= energy of 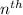 orbit
orbit
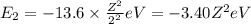

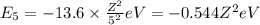
 (absorption)
(absorption) (emission)
(emission) (emission)
(emission)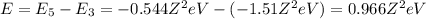 (absorption)
(absorption)

