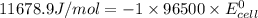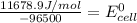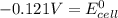Chemistry, 26.12.2019 23:31 BurwinkelElla19
Calculate the standard cell potential (e∘) for the reaction x(s)+y+(aq)→x+(aq)+y(s) if k = 8.97×10−3. express your answer to three significant figures and include the appropriate units. view available hint(s)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:10
Here’s one way to follow the scientific method. place the missing steps in the correct position in the process
Answers: 1

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2

Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2
You know the right answer?
Calculate the standard cell potential (e∘) for the reaction x(s)+y+(aq)→x+(aq)+y(s) if k = 8.97×10−3...
Questions


Chemistry, 12.01.2020 04:31



Mathematics, 12.01.2020 04:31



Mathematics, 12.01.2020 05:31




History, 12.01.2020 05:31

Mathematics, 12.01.2020 05:31

Mathematics, 12.01.2020 05:31

Mathematics, 12.01.2020 05:31



Mathematics, 12.01.2020 05:31

English, 12.01.2020 05:31

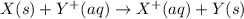 is -0.121 V
is -0.121 V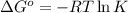
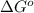 = Standard Gibbs free energy = ?
= Standard Gibbs free energy = ?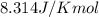
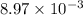
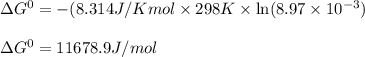
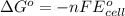
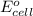 = standard cell potential = ?
= standard cell potential = ?