

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asample of neon occupies a volume of 375 ml at stp. what will be the volume of neon if the pressure is reduced to 90.0 kpa? a. 422 ml b. 422 l c. 333 ml d. 333 l
Answers: 2

Chemistry, 23.06.2019 04:20
Calculate the mass of 0.750 mol of the following substance. na3po4.
Answers: 1

Chemistry, 23.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 4.20 mol fe and 6.70 mol nio(oh) react?
Answers: 3

You know the right answer?
How many g of aluminum are needed to react completely with 4.40 gram of mn304, according to the chem...
Questions



Arts, 03.02.2021 05:10

Mathematics, 03.02.2021 05:10

Mathematics, 03.02.2021 05:10

Physics, 03.02.2021 05:10

Mathematics, 03.02.2021 05:10

Chemistry, 03.02.2021 05:10



Mathematics, 03.02.2021 05:10



Mathematics, 03.02.2021 05:10

Biology, 03.02.2021 05:10


Mathematics, 03.02.2021 05:10


Mathematics, 03.02.2021 05:10

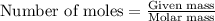 .....(1)
.....(1)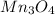 = 4.40 g
= 4.40 g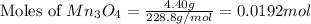
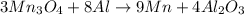
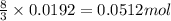 of aluminium
of aluminium


