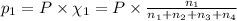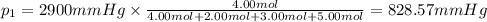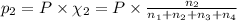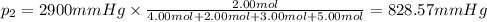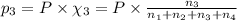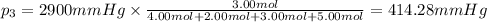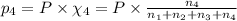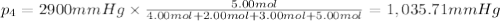Chemistry, 27.12.2019 02:31 ahmedeldyame
Amixture of gases consists of 4.00 moles of he, 2.00 moles of h2, 3.00 moles of co2 and 5.00 moles of ar. the total pressure of the mixture is 2900 mm. determine the mole fraction of each gas in the mixture. determine the mole percent of each gas in the mixture. determine the partial pressure of each gas in the mixture.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 11:00
Which are examples of how technology has advanced scientific understanding.1using hot water to sterilize medical equipment.2transplanting a human organ into another individual.3inserting genes from one sheep into another cell to make a cloneunderstanding the different structures that make up a cell.4examining microorganisms from the deepest parts of the ocean
Answers: 2

Chemistry, 23.06.2019 01:30
Which statement accurately represents the arrangement of electrons in bohr’s atomic model?
Answers: 2

Chemistry, 23.06.2019 06:20
Why is it that 85.48 rounded to two significant figures is 85 and not 86?
Answers: 1
You know the right answer?
Amixture of gases consists of 4.00 moles of he, 2.00 moles of h2, 3.00 moles of co2 and 5.00 moles o...
Questions


Mathematics, 12.10.2019 05:30

Health, 12.10.2019 05:30








Biology, 12.10.2019 05:30





History, 12.10.2019 05:30




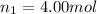
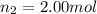
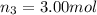
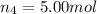
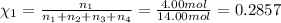
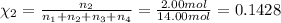

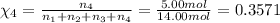
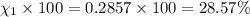
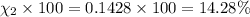
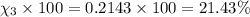
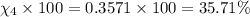
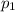
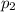
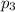
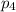
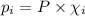
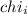 = Mole fraction of ith component
= Mole fraction of ith component