
Consider four different samples: aqueous licl, molten licl, aqueous agcl, and molten agcl. current run through each sample produces one of the following products at the cathode: solid lithium, solid silver, or hydrogen gas. match each sample to its cathodic product.
drag the appropriate items to their respective bins.
bin 1) solid lithium
bin 2) solid silver
bin 3) hydrogen gas

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1


Chemistry, 23.06.2019 03:00
Use the half-reactions of the reaction au(oh)3 + hi -> au +i2 +h2o to answer the questions
Answers: 1

You know the right answer?
Consider four different samples: aqueous licl, molten licl, aqueous agcl, and molten agcl. current...
Questions

Mathematics, 09.11.2019 23:31






Mathematics, 09.11.2019 23:31




Biology, 09.11.2019 23:31



Mathematics, 09.11.2019 23:31

Social Studies, 09.11.2019 23:31

Mathematics, 09.11.2019 23:31


Chemistry, 09.11.2019 23:31

Mathematics, 09.11.2019 23:31

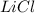
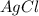
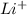 and
and 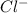 ions , and since its aqueous , it also contains
ions , and since its aqueous , it also contains 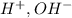 ions.But we know that , lithium is small in size and has a positive charge. Thus it has high polarizing power. Thus it cannot move easily through water. Even if it does , it reacts with
ions.But we know that , lithium is small in size and has a positive charge. Thus it has high polarizing power. Thus it cannot move easily through water. Even if it does , it reacts with 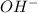 to form
to form 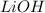 .
.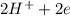 ⇒
⇒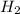
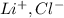 ions. Thus the product at cathode would be :
ions. Thus the product at cathode would be :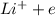 ⇒
⇒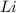
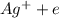 ⇒
⇒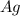 .....0.80V
.....0.80V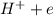 ⇒
⇒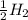 ....0.00V
....0.00V

