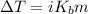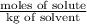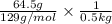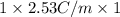Chemistry, 27.12.2019 03:31 douglasally
Assuming ideal behavior, calculate the boiling point in c of solution that contains 64.5g or the non-volatile non-electrolyte quinoline( molar mass= 129g/mole) in 500 grams of benzene. for benzene the normal boling point is 80.10 c and kb = 2.53c/m

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:50
Select the correct answer how does the heat content of the reaction change in the process of photosynthesis when a glucose molecule is formed? ca the value of is negative the value of qis positive the value of a remains constant the value of a decreases the value of equals zero e
Answers: 2


Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1
You know the right answer?
Assuming ideal behavior, calculate the boiling point in c of solution that contains 64.5g or the non...
Questions

English, 19.05.2020 16:22

Mathematics, 19.05.2020 16:22


World Languages, 19.05.2020 16:22

Mathematics, 19.05.2020 16:22

Mathematics, 19.05.2020 16:22

Mathematics, 19.05.2020 16:22



Mathematics, 19.05.2020 16:22

Biology, 19.05.2020 16:22




Spanish, 19.05.2020 16:22

Mathematics, 19.05.2020 16:22




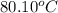 ,
, 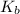 = 2.53 C/m
= 2.53 C/m