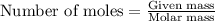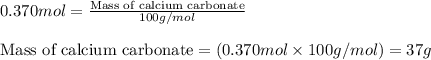Chemistry, 27.12.2019 04:31 Frankie103947
Ancient romans built often out of bricks and mortar. a key ingredient in their mortar was quicklime (calcium oxide), which they produced by roasting limestone (calcium carbonate).write a balanced chemical equation, including physical state symbols, for the decomposition of solid calcium carbonate (caco_3) into solid calcium oxide and gaseous carbon dioxide. caco3 → cao + co2 suppose 18.0 l of carbon dioxide gas are produced by this reaction, at a temperature of 320.0 degree c and pressure of exactly 1-atm. calculate the mass calcium carbonate that must have reacted. be sure your answer has the correct number of significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1

You know the right answer?
Ancient romans built often out of bricks and mortar. a key ingredient in their mortar was quicklime...
Questions

Mathematics, 05.02.2021 19:30


Social Studies, 05.02.2021 19:30



Mathematics, 05.02.2021 19:30

Social Studies, 05.02.2021 19:30




Spanish, 05.02.2021 19:30




Physics, 05.02.2021 19:30

Mathematics, 05.02.2021 19:30

Mathematics, 05.02.2021 19:30

Business, 05.02.2021 19:30

English, 05.02.2021 19:30

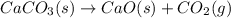
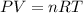
![320^oC=[320+273]K=593K](/tpl/images/0434/3992/aa925.png)
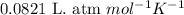
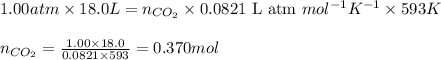
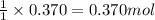 of calcium carbonate
of calcium carbonate