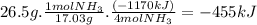Chemistry, 27.12.2019 04:31 mstahuggoo6102
Ammonia undergoes combustion to yield nitric oxide and water by the following reaction equation: 4 nh3 (g) + 5 o2 (g) → 4 no (g) + 6 h2o (g) δh = - 1170 kjif 26.5 g of nh3 is reacted with excess o2, what will be the amount of heat given off?

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 10:10
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1
You know the right answer?
Ammonia undergoes combustion to yield nitric oxide and water by the following reaction equation: 4...
Questions


Chemistry, 07.12.2021 16:50



Chemistry, 07.12.2021 16:50

Mathematics, 07.12.2021 16:50


Social Studies, 07.12.2021 16:50



English, 07.12.2021 16:50

Physics, 07.12.2021 16:50


English, 07.12.2021 16:50


Mathematics, 07.12.2021 16:50