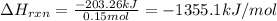Chemistry, 27.12.2019 04:31 liljohnsjs218
Asample of ethanol (c2h5oh), weighing 6.83 g underwent combustion in a bomb calorimeter by the following reaction: c2h5oh (l) + 3 o2 (g) → 2 co2 (g) + 3 h2o (l)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1
You know the right answer?
Asample of ethanol (c2h5oh), weighing 6.83 g underwent combustion in a bomb calorimeter by the follo...
Questions


Business, 12.12.2020 16:40

Mathematics, 12.12.2020 16:40






Mathematics, 12.12.2020 16:40

History, 12.12.2020 16:40


English, 12.12.2020 16:40




Mathematics, 12.12.2020 16:40

Mathematics, 12.12.2020 16:40


Mathematics, 12.12.2020 16:40

Biology, 12.12.2020 16:40

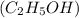 , weighing 6.83 g underwent combustion in a bomb calorimeter by the following reaction:
, weighing 6.83 g underwent combustion in a bomb calorimeter by the following reaction: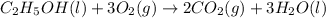
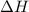 of the reaction?
of the reaction?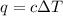
 = change in temperature =
= change in temperature = 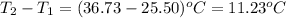
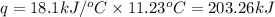
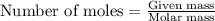
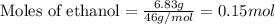
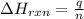
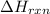 = enthalpy change of the reaction
= enthalpy change of the reaction