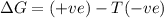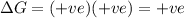Acertain process has δh° > 0, δs° < 0, and δg° > 0. the values of δh° and δs° do not depend on the temperature. which of the following is a correct conclusion about this process? none of the above conclusions is correct. it is non-spontaneous at all t. it is spontaneous at low t. it is spontaneous at all t. it is spontaneous at high t

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
50 pts plz what is the physical state of matter of baking soda.
Answers: 1

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2

You know the right answer?
Acertain process has δh° > 0, δs° < 0, and δg° > 0. the values of δh° and δs° do not dep...
Questions

Mathematics, 18.09.2020 08:01

Mathematics, 18.09.2020 08:01

Mathematics, 18.09.2020 08:01

History, 18.09.2020 08:01

English, 18.09.2020 08:01

Biology, 18.09.2020 08:01

Chemistry, 18.09.2020 08:01

Mathematics, 18.09.2020 08:01

Mathematics, 18.09.2020 08:01

Mathematics, 18.09.2020 08:01

Mathematics, 18.09.2020 08:01

Mathematics, 18.09.2020 08:01

Mathematics, 18.09.2020 08:01

Mathematics, 18.09.2020 08:01

Mathematics, 18.09.2020 08:01

Mathematics, 18.09.2020 08:01

Mathematics, 18.09.2020 08:01

Arts, 18.09.2020 08:01

Mathematics, 18.09.2020 08:01

Mathematics, 18.09.2020 08:01

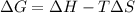
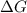 = Gibbs free energy = +ve
= Gibbs free energy = +ve
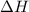 = enthalpy change = +ve
= enthalpy change = +ve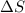 = entropy change = -ve
= entropy change = -ve