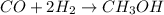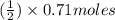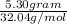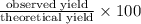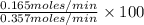Chemistry, 27.12.2019 05:31 lorelei7668
Methanol (ch3oh) can be produced by the following reaction: co(g) 1 2h2(g) 88n ch3oh(g) hydrogen at stp flows into a reactor at a rate of 16.0 l/min. carbon monoxide at stp flows into the reactor at a rate of 25.0 l/min. if 5.30 g of methanol is produced per minute, what is the percent yield of the reaction?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3


Chemistry, 23.06.2019 06:00
Complete the sentences to best explain the ranking.match the words below to the appropriate blanks in the sentences.a less polar bondhigher molar massion-dipole forcesstronger intermolecular forcesdipole-dipole forcesdispersion forceshydrogen bonding1. h2s and h2se exhibit the following intermolecular forces:.2. therefore, when comparing h2s and h2se the one with a has a higher boiling point .3. the strongest intermolecular force exhibited by h2o is . therefore, when comparing h2se and h2o the one with has a higher boiling point.
Answers: 1
You know the right answer?
Methanol (ch3oh) can be produced by the following reaction: co(g) 1 2h2(g) 88n ch3oh(g) hydrogen at...
Questions

Physics, 19.03.2022 14:00








Biology, 19.03.2022 14:00

English, 19.03.2022 14:00


Mathematics, 19.03.2022 14:00



History, 19.03.2022 14:00


Social Studies, 19.03.2022 14:00

Mathematics, 19.03.2022 14:00

Geography, 19.03.2022 14:00

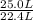 per minute
per minute
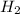 being fed per minute =
being fed per minute = 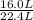 per minute
per minute