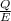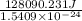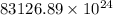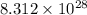Chemistry, 27.12.2019 05:31 MiddleSchool2005
Acontainer with 0.389 l of water is placed in a microwave and radiated with electromagnetic energy with a wavelength of 12.9 cm. the temperature of the water rose by 78.7 °c. calculate the number of photons that were absorbed by the water. assume water has a density of 1.00 g ⋅ ml − 1 and a specific heat of 4.184 j ⋅ g − 1 ⋅ ° c − 1 .

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which feature do highland climates have that lower elevation areas do not?
Answers: 1

Chemistry, 22.06.2019 03:00
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 06:00
What type of electromagnetic radiation has a shorter wavelength than blue light
Answers: 2

Chemistry, 22.06.2019 07:00
6what is the importance of water on earth? a) it keeps the top layer of the geosphere cool b) it allows life to exist c) it provides ice at the poles d) it creates earth's blue color from space
Answers: 2
You know the right answer?
Acontainer with 0.389 l of water is placed in a microwave and radiated with electromagnetic energy w...
Questions

English, 27.06.2019 21:40

Mathematics, 27.06.2019 21:40

Chemistry, 27.06.2019 21:40

Mathematics, 27.06.2019 21:40

Mathematics, 27.06.2019 21:40

Mathematics, 27.06.2019 21:40

Physics, 27.06.2019 21:40

Biology, 27.06.2019 21:40

Chemistry, 27.06.2019 21:40

Mathematics, 27.06.2019 21:40

Biology, 27.06.2019 21:40

Biology, 27.06.2019 21:40

Social Studies, 27.06.2019 21:40







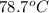
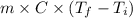
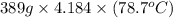
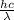
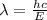
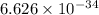 J s
J s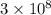 m/s
m/s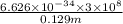
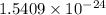 J/photons
J/photons