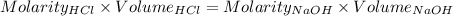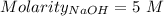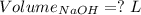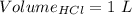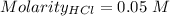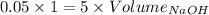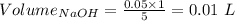Chemistry, 27.12.2019 05:31 svarner2001
Consider a solution that is 0.05 m hcl. your goal is to neutralize 1 l of this solution (i. e. bring the ph to 7). you also have a solution that is 5 m naoh. what volume of this solution should you add to the hcl solution, to neutralize it? provide your answer in units of liters (l).

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 23:30
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2

Chemistry, 23.06.2019 01:30
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
Consider a solution that is 0.05 m hcl. your goal is to neutralize 1 l of this solution (i. e. bring...
Questions

Social Studies, 03.11.2020 01:50




Mathematics, 03.11.2020 01:50

History, 03.11.2020 01:50

History, 03.11.2020 01:50

Mathematics, 03.11.2020 01:50

English, 03.11.2020 01:50




Mathematics, 03.11.2020 01:50

Mathematics, 03.11.2020 01:50



Biology, 03.11.2020 01:50

History, 03.11.2020 01:50


English, 03.11.2020 01:50

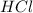 = Moles of NaOH
= Moles of NaOH