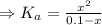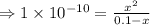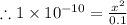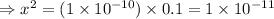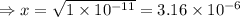Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Select the correct text in the passage. which sentences describe examples of sustainable living? i live in an old apartment building downtown, but my company is based in an office park on the outskirts of the city. i drive an old car that needs to be replaced. i plan to buy a hybrid for better gas mileage, but for now i am able to carpool with a couple of friends from work. the drive to the office park is about 45 minutes each way, but we do get to work in a modern building. the architects just received a leed certification for the design.
Answers: 3

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 19:00
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1

Chemistry, 22.06.2019 22:00
8) warming your hands by a fire is an example if which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 1
You know the right answer?
Bh+clo4- is a salt formed from the base b (kb = 1.00e-4) and perchloric acid. it dissociates into bh...
Questions

English, 07.12.2021 14:00

Mathematics, 07.12.2021 14:00

Social Studies, 07.12.2021 14:00

Mathematics, 07.12.2021 14:00

English, 07.12.2021 14:00

History, 07.12.2021 14:00

Mathematics, 07.12.2021 14:00



Mathematics, 07.12.2021 14:00


Mathematics, 07.12.2021 14:00

World Languages, 07.12.2021 14:00

Mathematics, 07.12.2021 14:00

Computers and Technology, 07.12.2021 14:00

Mathematics, 07.12.2021 14:00


History, 07.12.2021 14:00


Geography, 07.12.2021 14:00

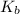 = 1 × 10⁻⁴, Concentration of salt: BH⁺ClO₄⁻ = 0.1 M
= 1 × 10⁻⁴, Concentration of salt: BH⁺ClO₄⁻ = 0.1 M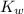 = 1 × 10⁻¹⁴
= 1 × 10⁻¹⁴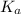 ) for the weak acid (BH⁺) can be calculated by the equation:
) for the weak acid (BH⁺) can be calculated by the equation: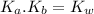
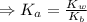
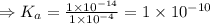
![K_{a} = \frac{\left [B \right ] \left [H_{3}O^{+}\right ]}{\left [BH^{+} \right ]} = \frac{(x)(x)}{(0.1 - x)} = \frac{x^{2}}{0.1 - x}](/tpl/images/0434/5359/b792d.png)