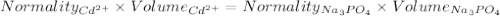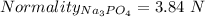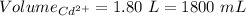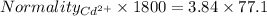Chemistry, 28.12.2019 00:31 yupthatsme2121
A1.80 l water sample is thought to contain cadmium ions. if 26.00 g cadmium phosphate (527.2 g/mol) precipitates when 77.1 ml of a 1.28 m sodium phosphate solution is added to the water sample, what is the molar concentration of cd in the original water sample?
a. 0.148 m
b. 0.164 m
c. 0.0548 m
d. 0.0493 m
e. 0.0822 m

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Explain why scientists use shared characteristics to make cladograms.
Answers: 1

Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2
You know the right answer?
A1.80 l water sample is thought to contain cadmium ions. if 26.00 g cadmium phosphate (527.2 g/mol)...
Questions


Social Studies, 30.11.2019 21:31






Mathematics, 30.11.2019 21:31


Mathematics, 30.11.2019 21:31


English, 30.11.2019 21:31

Health, 30.11.2019 21:31


Mathematics, 30.11.2019 22:31



Physics, 30.11.2019 22:31


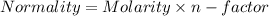
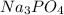 = 1.28 M
= 1.28 M
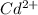 = Gram equivalents of
= Gram equivalents of