
Chemistry, 28.12.2019 00:31 quincyjosiah07
How much heat is absorbed/released when 20.00 g of nh3(g) reacts in the presence of excess o2 (g) to produce no (g) and h2o (l) according to the following chemical equation? 4nh3 (g) + 5o2 (g) > 4no (g) +6h2o (l) δ h: +1168 kja. 342.9 kj of heat are absorbed. b. 342.9 kj of heat are released. c. 1372 kj of heat are absorbed. d. 1372 kj of heat are released.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which of the dna typing techniques do you think you would choose if you had to analyze a dna sample? why?
Answers: 1

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2


Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3
You know the right answer?
How much heat is absorbed/released when 20.00 g of nh3(g) reacts in the presence of excess o2 (g) to...
Questions

Mathematics, 01.09.2019 17:20

Mathematics, 01.09.2019 17:20





History, 01.09.2019 17:20


Computers and Technology, 01.09.2019 17:20



History, 01.09.2019 17:20


History, 01.09.2019 17:20

Geography, 01.09.2019 17:20


Computers and Technology, 01.09.2019 17:20


Spanish, 01.09.2019 17:20

Social Studies, 01.09.2019 17:20

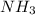 as:-
as:-
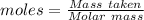
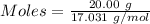
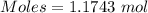
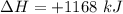
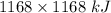 of heat
of heat

