
Chemistry, 28.12.2019 01:31 hrijaymadathil
Areaction a(aq)+b(aq)↽−−⇀c(aq) has a standard free‑energy change of −4.51 kj/mol at 25 °c.
what are the concentrations of a, b, and c at equilibrium if, at the beginning of the reaction, their concentrations are 0.30 m, 0.40 m, and 0 m, respectively?
how would your answers change if the reaction had a standard free-energy change of +4.51 kj/mol?
a. all concentrations would be lower.
b. there would be more a and b but less c.
c. there would be less a and b but more c.
how would your answers change if the reaction had a standard free-energy change of +4.51 kj/mol?
a. all concentrations would be lower.
b. there would be more a and b but less c.
c. there would be less a and b but more c.
d. there would be no change to the answers.
e. all concentrations would be higher.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1


Chemistry, 23.06.2019 06:00
Is the flow of energy during vaporizing more like the flow during melting or during freezing
Answers: 1

You know the right answer?
Areaction a(aq)+b(aq)↽−−⇀c(aq) has a standard free‑energy change of −4.51 kj/mol at 25 °c.
wha...
wha...
Questions


Mathematics, 20.05.2020 19:00

Chemistry, 20.05.2020 19:00

Arts, 20.05.2020 19:00



History, 20.05.2020 19:01

Mathematics, 20.05.2020 19:01

History, 20.05.2020 19:01

English, 20.05.2020 19:01


Biology, 20.05.2020 19:01

Mathematics, 20.05.2020 19:01



Physics, 20.05.2020 19:01


Computers and Technology, 20.05.2020 19:01

French, 20.05.2020 19:01

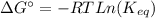 (4)
(4)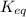 : is the equilibrium constant
: is the equilibrium constant 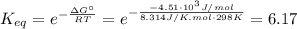 (5)
(5) ![K_{eq} = \frac{[C]}{[A][B]} = \frac{x}{(0.3 - x)(0.4 - x)}](/tpl/images/0435/1489/3c26a.png) (6)
(6) ![[A] = 0.3 - x_{2} = 0.3 - 0.17 = 0.13 M](/tpl/images/0435/1489/8007b.png)
![[B] = 0.4 - x_{2} = 0.4 - 0.17 = 0.23 M](/tpl/images/0435/1489/c1e68.png)
![[C] = x = 0.17 M](/tpl/images/0435/1489/fb724.png)
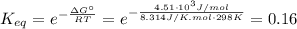
![[A] = 0.3 - x_{2} = 0.3 - 0.017 = 0.28 M](/tpl/images/0435/1489/5f84a.png)
![[B] = 0.4 - x_{2} = 0.4 - 0.017 = 0.38 M](/tpl/images/0435/1489/7a756.png)
![[C] = x = 0.017 M](/tpl/images/0435/1489/39608.png)


