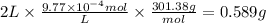Chemistry, 28.12.2019 01:31 ronniethefun
The drug dobutamine (fw=301.38g/mol, c18h23no3c18h23n03) has a molar absorptivity of 703 at 262nm. one tablet is dissolved in water and diluted to a volume of 2l. if the solution exhibits an absorbance of 0.687 in the uv region at 262nm in a 1- cm cell, how many grams to dobutamine are contained in the tablet? a. 0.5277g b. 0.58889 c. 9.77x 10g d. 97.778ge. 9.7778g

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2

Chemistry, 22.06.2019 20:10
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1
You know the right answer?
The drug dobutamine (fw=301.38g/mol, c18h23no3c18h23n03) has a molar absorptivity of 703 at 262nm. o...
Questions


Mathematics, 17.09.2021 18:40




Mathematics, 17.09.2021 18:40




Mathematics, 17.09.2021 18:40

Mathematics, 17.09.2021 18:40

Computers and Technology, 17.09.2021 18:40

Mathematics, 17.09.2021 18:40


Mathematics, 17.09.2021 18:40

Computers and Technology, 17.09.2021 18:40


Mathematics, 17.09.2021 18:40

Mathematics, 17.09.2021 18:40

Mathematics, 17.09.2021 18:40