Consider the following standard heats of formation:
p₄o₁₀(s) = -3110 kj/mol
h₂o(l) =...


Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 23.06.2019 04:20
The lewis diagrams for magnesium and fluorine are shown below. what is the correct chemical formula for magnesium fluoride? a. mgf b. mg2f c. mgf2 d. mg2f3
Answers: 1

Chemistry, 23.06.2019 07:00
Agas has an empirical formula ch4. 0.16g of the gas occupies a volume of 240cm^3 what is the molecular formula of the me anyone who !
Answers: 1
You know the right answer?
Questions





History, 21.07.2019 03:00

Geography, 21.07.2019 03:00

Mathematics, 21.07.2019 03:00



Mathematics, 21.07.2019 03:00

History, 21.07.2019 03:00

Health, 21.07.2019 03:00


Biology, 21.07.2019 03:00

Biology, 21.07.2019 03:00

Biology, 21.07.2019 03:00

Social Studies, 21.07.2019 03:00


Social Studies, 21.07.2019 03:00

Mathematics, 21.07.2019 03:00

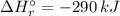
![\Delta H_{f}^{\circ } [P_{4}O_{10}(s)]](/tpl/images/0435/2502/5b047.png) = -3110 kJ/mol,
= -3110 kJ/mol,![\Delta H_{f}^{\circ } [H_{2}O(l)]](/tpl/images/0435/2502/46f61.png) = -286 kJ/mol,
= -286 kJ/mol, ![\Delta H_{f}^{\circ } [H_{3}PO_{4}(s)]](/tpl/images/0435/2502/1d826.png) = -1279 kJ/mol
= -1279 kJ/mol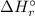 = ?
= ?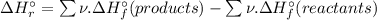
![\Delta H_{r}^{\circ } = [4 \times \Delta H_{f}^{\circ } [H_{3}PO_{4}(s)]] - [1 \times \Delta H_{f}^{\circ } [P_{4}O_{10}(s)] + 6 \times \Delta H_{f}^{\circ } [H_{2}O(l)]]](/tpl/images/0435/2502/c9ebf.png)
![\Rightarrow \Delta H_{r}^{\circ } = [4 \times (-1279\, kJ/mol)] - [1 \times (-3110\, kJ/mol) + 6 \times (-286\, kJ/mol)]](/tpl/images/0435/2502/ea7a2.png)
![\Rightarrow \Delta H_{r}^{\circ } = [-5116\, kJ] - [-3110\, kJ -1716\, kJ]](/tpl/images/0435/2502/7e6ce.png)
![\Rightarrow \Delta H_{r}^{\circ } = [-5116\, kJ] - [-4826\, kJ] = -290\,kJ](/tpl/images/0435/2502/ee612.png)


