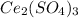Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 23:00
The agent of mechanical weathering in which rock is worn away by the grinding action of other rock particles is call
Answers: 1

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1
You know the right answer?
Substances with high lattice energies tend to be less soluble than substances with low lattice energ...
Questions





Mathematics, 26.11.2021 16:50

Arts, 26.11.2021 16:50

Social Studies, 26.11.2021 16:50

Social Studies, 26.11.2021 16:50



Mathematics, 26.11.2021 17:00

Social Studies, 26.11.2021 17:00



World Languages, 26.11.2021 17:00

Social Studies, 26.11.2021 17:00

Chemistry, 26.11.2021 17:00

Arts, 26.11.2021 17:00

Business, 26.11.2021 17:00

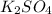 >
>