
Chemistry, 28.12.2019 02:31 appattuvilai1234
Solid copper (ii) oxide is placed in a sealed container with an excess of ammonia gas. when equilibrium is established the partial pressure of n2 is 0.255atm. the reaction is as follows: 3cuo (s) + 2 nh3 (g) ↔ 3 cu (s) + n2 (g) + 3 h2o (g) a) what is the partial pressure of h2o (g) at equilibrium?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1

Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2

Chemistry, 22.06.2019 11:00
Surface currents are caused by blank space . question 14 options: surface currents are caused by? differences in water temperature high salinity differences in density wind forces
Answers: 1

Chemistry, 23.06.2019 01:00
The primary products of complete combustion of fossil fuels are a. carbon dioxide and water b. methane and water c. carbon monoxide and water d. carbon dioxide and carbon monoxide
Answers: 1
You know the right answer?
Solid copper (ii) oxide is placed in a sealed container with an excess of ammonia gas. when equilibr...
Questions

Advanced Placement (AP), 29.03.2021 22:50

Mathematics, 29.03.2021 22:50

English, 29.03.2021 22:50

Mathematics, 29.03.2021 22:50







Mathematics, 29.03.2021 22:50

Mathematics, 29.03.2021 22:50

Biology, 29.03.2021 22:50


Mathematics, 29.03.2021 22:50


Mathematics, 29.03.2021 22:50


History, 29.03.2021 22:50

History, 29.03.2021 22:50

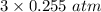 = 0.765 atm
= 0.765 atm

