
Chemistry, 28.12.2019 03:31 camila9022
Determine the heat of reaction (δhrxn) for the combustion of ethanol (c2h5oh) by using heat of formation data: c2h5oh (l) + 3 o2 (g) → 2 co2 + 3 h2o (g)

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 22.06.2019 21:30
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1

You know the right answer?
Determine the heat of reaction (δhrxn) for the combustion of ethanol (c2h5oh) by using heat of forma...
Questions




Geography, 23.05.2021 20:40

Mathematics, 23.05.2021 20:40


Spanish, 23.05.2021 20:40


English, 23.05.2021 20:40


Physics, 23.05.2021 20:40

Physics, 23.05.2021 20:40

Mathematics, 23.05.2021 20:40

Mathematics, 23.05.2021 20:40




English, 23.05.2021 20:40


Mathematics, 23.05.2021 20:40

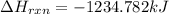
![\Delta H_{rxn}=\sum [n_{i}\times \Delta H_{f}^{0}(product)_{i}]-\sum [n_{j}\times \Delta H_{f}^{0}(reactant_{j})]](/tpl/images/0435/2901/43912.png)
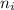 and
and 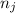 are number of moles of product and reactant respectively (equal to their stoichiometric coefficient).
are number of moles of product and reactant respectively (equal to their stoichiometric coefficient).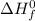 is standard heat of formation.
is standard heat of formation.![\Delta H_{rxn}=[2mol\times \Delta H_{f}^{0}(CO_{2})_{g}]+[3mol\times \Delta H_{f}^{0}(H_{2}O)_{g}]-[1mol\times \Delta H_{f}^{0}(C_{2}H_{5}OH)_{l}]-[3mol\times \Delta H_{f}^{0}(O_{2})_{g}]](/tpl/images/0435/2901/adad7.png)
![\Delta H_{rxn}=[2mol\times -393.509kJ/mol]+[3mol\times -241.818kJ/mol]-[1mol\times -277.69kJ/mol]-[3mol\times 0kJ/mol]](/tpl/images/0435/2901/6491b.png)


