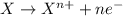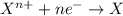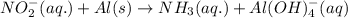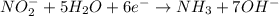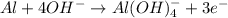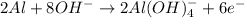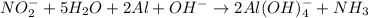Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Margaret wants to make an orange flavored drink by stirring powdered drink mix into a glass of water. she doesn't like drinks that have small clumps of powdered solid in them, so she wants the drink to be a perfect solution. what factors should margaret not consider when deciding how much powder to add to her glass of water?
Answers: 3

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2
You know the right answer?
If you complete and balance the following oxidation-reduction reaction in basic solutionno2−(aq) + a...
Questions

Biology, 26.10.2020 21:50



English, 26.10.2020 21:50


Mathematics, 26.10.2020 21:50



History, 26.10.2020 21:50

Mathematics, 26.10.2020 21:50




Mathematics, 26.10.2020 21:50

Mathematics, 26.10.2020 21:50

Mathematics, 26.10.2020 21:50

Advanced Placement (AP), 26.10.2020 21:50

Mathematics, 26.10.2020 21:50

Mathematics, 26.10.2020 21:50

Chemistry, 26.10.2020 21:50